Building a Robust Laboratory Quality Management (LQM) Program for Analytical Testing

Analytical testing laboratories operate within a complex system inherently prone to variability. Maintaining quality results necessitates rigorous assessment and control of this variability. While Good Laboratory Practices (GLPs), Total Quality Management (TQM), and ISO 9000 quality systems have been implemented, a comprehensive Laboratory Quality Management (LQM) program offers a more effective solution. An LQM system manages both technical and non-technical factors influencing laboratory variability, leading to reduced analytical variation, increased efficiency, lower costs, and enhanced confidence in results.
This blog post will guide you through building a successful LQM program for your laboratory.
Our Scientists
A team of more than 100 veteran chemists, biologists, microbiologists, food scientists, stability scientists and sensory scientists handles every aspect of our service. Learn more about Medallion Labs commitment to quality.
Learn More About UsSix Crucial Components of an LQM Program
Most LQM systems are built upon the framework of ISO/IEC 17025, supplemented by guidelines from organizations like the Association of Official Analytical Chemists International (AOAC International) and the American Council of Independent Laboratories (ACIL). These organizations agree on key components for a robust LQM program:
1. Quality Manual
A comprehensive document detailing the laboratory's quality system, including management and technical policies (detailed below). Version control is crucial to prevent the use of outdated manuals.
2. Staff and Training
All personnel must be competent and receive documented training from qualified instructors. Consistent training from a single source is recommended.
3. Methodology
Methods must be consistently formatted, validated to ensure acceptable precision and accuracy, and readily accessible to all users. Methods can be internally developed or sourced from reputable organizations. Imported methods require verification of proper performance within the laboratory.
4. Reference Materials (In-House or Recognized)
In-house reference materials (IHRMs) are invaluable. An IHRM is a test matrix with a known analyte concentration, analyzed with each batch. This helps:
- Determine method control for each run.
- Understand analytical method variation.
- Build confidence in results.
The cost of reruns due to out-of-control situations should be weighed against the cost of reporting inaccurate results. IHRMs should be representative of typical matrices, contain analyte levels comparable to those normally tested, and be stable during storage (or have known storage characteristics).
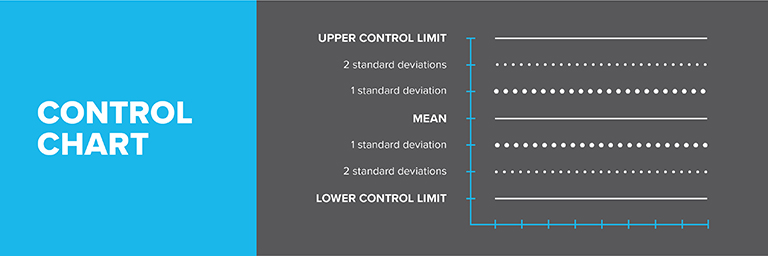
Choosing an IHRM
Analyze the IHRM at least 20 times, varying days, analysts, and points within the analytical run. Calculate the standard deviation (SD) and establish control limits:
Establishing Control Limits
Analyze the IHRM at least 20 times, varying days, analysts, and points within the analytical run. Calculate the standard deviation (SD) and establish control limits:
- Upper Control Limit (UCL) = mean + (3 x SD)
- Lower Control Limit (LCL) = mean – (3 x SD)
Monitor results on a control chart. An out-of-control situation is indicated by:
- Any point outside the LCL or UCL
- 4 out of 5 consecutive points outside ±1SD
- 2 out of 3 consecutive points outside ±2SD
- 8 consecutive points all above or below the mean
- 8 consecutive points all increasing or decreasing
- Non-random patterns
5. Record Keeping
Maintain detailed logs (standard preparation, calibration, analysis, training, equipment maintenance), and Corrective Action Protocol (CAP) records for out-of-control situations and instrument issues.
6. Considering Cost vs. Benefits
While initial implementation and potential reruns represent costs, the benefits—increased confidence, customer satisfaction, reduced waste, improved efficiency—far outweigh them.
Management and Technical Requirements (Based on ISO/IEC 17025)
The ISO/IEC 17025 standard outlines both management and technical requirements for LQM:
Management Requirements: Written policies are required for document control, management review, contracts, purchasing, complaint handling, non-conforming results, corrective actions, audits, and continuous improvement.
Technical Requirements: Policies should cover personnel selection and training, laboratory environmental conditions, test method validation, equipment and instrumentation, measurement traceability, method verification, sampling, and sample handling.
The Path Forward
A comprehensive LQM program is vital for analytical certification and contributes to accurate results, crucial in the food industry where consumers rely on nutrition label information. While IHRMs are highly recommended, other quality improvement tools include AACC and AOAC check sample programs, certified reference materials, and inter-laboratory collaborations. Adopting a robust LQM program is a strategic investment in cost reduction and quality enhancement.
Let's Get to Work!
Submit your order online and ship your samples today. If you have questions, we are always here to help.
Medallion Labs+
A food testing program designed with mid-market and enterprise food and ingredient manufacturers in mind.
- Deneen Rief, John Szpylka, Ph.D., Jon DeVries, Ph.D. 1998 Assuring Quality Analytical Results Through the Use of In-House Reference Materials. Poster given at the AOAC Annual meeting September,1998.
- To view the ACIL/FLAWG proposal, visit: www.aoac.org/techprog/chem.htm